- Visibility 102 Views
- Downloads 18 Downloads
- DOI 10.18231/j.ijce.2022.013
-
CrossMark
- Citation
Evaluation of physico -mechanical properties of graphene nanoparticles modified nano hybrid composite resin after thermo mechanical loading cycle - An in vitro study
- Author Details:
-
Madhumita S *
-
Dhanavel Chakravarthy
-
Viajayaraja S
Introduction
Composite resins are considered the material of choice in restorative dentistry because of the increasing demand for high-quality aesthetic results.[1] These materials have been the focus of a great deal of research in recent years to ensure the long-term clinical success of the restoration. Despite the continuous evolution of these resins high polymerization shrinkage and lack of fracture toughness render their clinical success relatively shorter.[2] In an attempt to offset the existing shortcomings of traditional dental composites, the resins have undergone progressive evolution, from the conventional composites reinforced with strong filler particles to the relatively newer micro-filled and hybrid composites.[3]
Recently the application of the Science of Nanotechnology has invaded the field of dentistry in an attempt to substantially improve the mechanical, aesthetic, and optical properties of the restorations. [4] This technology allows the addition of a greater amount of Nanosized filler particles like Silver, Zinc, Titanium Dioxide, Zirconia, Silica, and Graphene that are compatible with the composite to be added into the Composite resin matrix.[5], [6], [7], [8] Graphene Nano Particles (GNP) are novel fillers that possess a high fracture strength, mechanical strength, chemical stability, a large surface area, flexibility and are also biocompatible and non-cytotoxic. Graphene is also capable of transferring stress across the interface.[9], [10] The increase in filler load is capable of reducing the polymerization shrinkage and increasing the Physico- mechanical properties like Microhardness, Surface roughness, Flexural strength of resin composites which determines its clinical longevity.[11]
The restoration should possess high wear resistance values so that wearing will not cause faulty tooth relationships which will affect aesthetics and masticatory efficiency.[12] Masticatory and other parafunctional forces can cause flexing, bending, and twisting forces that can cause permanent deformation of the restoration, so the restorative material must have high flexural strength.[13] For better clinical performance the restoration must not undergo surface degradation which may render the restorations surface rough, leading to discoloration, plague accumulation, and risk of secondary caries.[14], [15]
Most of the studies involving the addition of Nanoparticles to dental composites resins have mainly focused on their antibacterial effects. This study evaluated the Surface roughness, Microhardness, and the Flexural strength, of Nanohybrid composite resin modified with Graphene Nanoparticles (GNP). The study hypothesis was that there was no significant difference in evaluated physicomechanical properties of unmodified and Graphene nanoparticles modified Tetric N Ceram Nanohybrid composite resin.
Materials and Methods
Preparation of experimental composite
The present study evaluated the effect of Graphene nanoparticles (GNP) on the physico-mechanical properties of a Nano Hybrid composite resin. Nano Hybrid composite resin (Tetric N Ceram – Ivoclar Vivadent AG ) and Graphene nano powder (Adnano Technologies Pvt. Ltd) with 99% purity and particle size 10 µm was used in the study.
Graphene nano powder was weighed at 0.01% and 0.02% w/w to the Tetric N Ceram composite resin on electronic Micro Balance (Wensar HPB 201) with an accuracy of 0.0001g. The weighed Graphene oxide nanoparticles were added to the Tetric N Ceram composite resin and blended using a Sonicator. The mixed resin was transferred into black containers, labelled as CG1 (0.01%) and CG2 (0.02%), and stored in a dark container to prevent any undue polymerization before the start of the study. The unmodified composite resin was the control group C.
Specimen preparation
A total of 72 specimens were prepared for the two interventional and control groups for the present study. The specimen’s distribution is presented as a flowchart in [Figure 1].
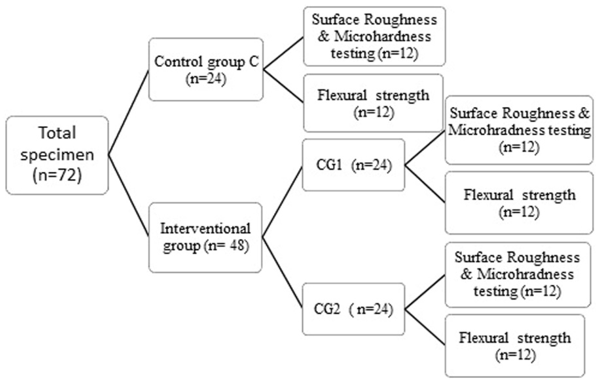
Preparation of specimens for Surface Roughness (SR and Microhardness (MR
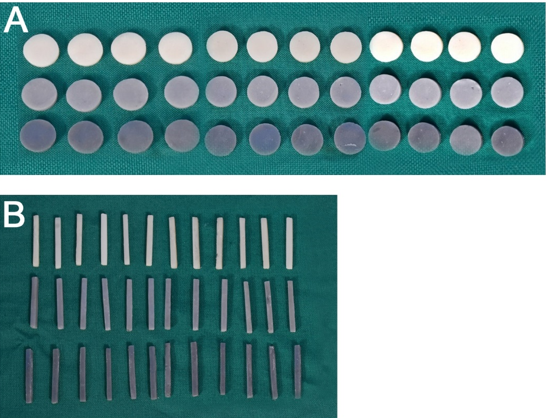
A Total of 36 Six round specimens (12.0 mm diameter×2.0 mm thickness) were fabricated, ie12 specimens were fabricated per each resin group (C, CG1 & CG2) using silicone mould ([Figure 2]A). The silicone mould was packed with the composite resin and light-cured according to the manufacturer’s recommendation on either end of the specimen using a visible light-cure unit (Ivoclar Blue phase NM). Once the packing was completed the last increment of material is covered by a mylar strip and compressed firmly so that there was a uniform distribution of the material and the excess material was expelled. The specimens were light-cured on either end of the specimen. The cured specimens were carefully removed from the mould and finished using Shofu Super Snap Mini kit CA. The dimensions of the finished specimens were checked using a Digital micro-meter electronic caliper set and polished using a soft prophy cup with a contact time of 15 seconds per specimen and were stored in de-ionized water at 37±10 C for 24 hours before the measurement. [Figure 2] Fabricated Specimens
Preparation of Specimen for Flexural Strength (FS)
A Total of 36 rectangular specimens (25 mm in length×2 mm in width×2 mm in height) were fabricated, ie 12 specimens per each resin group (C, C1 & C2) were fabricated using silicone mould ([Figure 2]B). The procedure of specimen preparation was similar to that of surface roughness and microhardness specimens and was stored in de-ionized water at 37±10 C for 24 hours before the measurement.
Thermal & mechanical loading cycling
To simulate aging in the oral cavity, the specimens of the C, CG1 and CG2 groups were subjected to Thermo-Mechanical cyclic loading. They were subjected to 2,00,000 mechanical cycles with an 86-N load at a 2 Hz frequency in a mechanical cycling machine (Instron Ltd, England). The specimens were then subjected to 550 cycles in a Custom- made Thermo cycling apparatus. It contains three water baths 50 ± 10 C; 370 ± 10 C; 550 ± 10 C, the specimens were subjected to 30 seconds per bath with an interval of 15 seconds.
Specimen testing
[Figure 3] Instruments

Surface roughness measurement
The surface roughness measurement was performed using a 3D Optical profilometer (MicroXAM-800) (Fig 3A). The Representative specimens were submitted to surface roughness testing at five different locations 1 mm away from each other to prevent overlapping of the testing. The average of the five readings was calculated and the surface roughness value was assigned to that particular specimen.
Microhardness measurement
The Microhardness test was performed using a microhardness tester Shimadzu (Asia Pacific Pvt Ltd, Model HMV -2T) (Fig 3B) with the magnification of 400 X equipped with Vickers diamond indenter. For each specimen, a 50 grams load was applied for 30 seconds to make a diamond-shaped indentation on the specimen. The indentations were performed on five different areas but not closer than 1 mm to the adjacent indentation or on the margins. The average of the five readings was calculated and microhardness values were assigned to that particular specimen.
Flexural strength measurement
The flexural strength test was performed using a universal testing machine (UNITEK -94100) (Fig 3C) in a three-point bending mode. The specimens were placed on two parallel supports positioned 20 mm apart. A weight of 2 kN was applied at a crosshead speed of 0.5 mm/min, and the maximum resistance to fracture was recorded in MPa. Flexural strength is calculated using the formula
Flexural Strength σ=3FL2BH2
F - is the maximum load (in newtons)
L - is the distance between the supports (in mm)
B - is the width of the specimen (in mm)
H - the height (also in mm).
Statistical analysis was done by Kruskal Wallis ANOVA and Pairwise Post hoc/ Multiple pairwise comparisons using Dunn's procedure, for intergroup comparison. In all the tests, ‘the p-value ≤0.05 was considered statistically significant.
Results
The Surface roughness, Microhardness & Flexural strength showed a statistically significant difference (p <0.001) between the control group C and interventional groups CG1 & CG2. The surface roughness measurements showed the highest mean value in the CG2 Group (0.185 ± 0.003) and the lowest mean value in the C Group (0.154 ± 0.001) Likewise, for the Microhardness measurement the highest mean value was observed in the CG1 group (94 ± 2.5) and the lowest value was observed in C Group (60.3 ± 1.0). The highest mean value for the flexural strength was observed in the CG1 Group (117.57 ± 6.46) and the lowest value was in the CG2 Group (77.55 ± 4.85). ([Table 1])
|
Groups |
Mean±SD |
||
|
Surface Roughness |
Micro-Hardness |
Flexural Strength |
|
|
C |
0.154 ± 0.001 |
60.3 ± 1.0 |
96.01 ± 4.73 |
|
CG1 |
0.165 ± 0.003 |
94.0 ± 2.5 |
117.57 ± 6.46 |
|
CG2 |
0.185 ± 0.003 |
85.5 ± 1.3 |
77.55 ± 4.85 |
|
K- Value |
31.26 |
31.19 |
31.37 |
|
p Value |
< 0.0001 |
< 0.0001 |
< 0.0001 |
|
Parameters tested |
|
CG1 |
CG2 |
|
Surface Roughness |
“p” value of Control Group C versus Interventional group CG1& CG2 |
0.05* |
<0.01 * |
|
Microhardness |
<0.01* |
0.04* |
|
|
Flexural strength |
0.05* |
0.01* |
The Post hoc analysis of Surface hardness, Microhardness, and flexural strength showed a statistically significant difference between the control Groups C and the two interventional groups CG1 and CG2. ([Table 2])
Discussion
Composite restorative materials represent the current state of the art in the field of restorative materials. It is one of the most accomplished contemporary biomaterials since they substitute biological tissue in both appearance and function. Recently the application of composite resin as direct restorations in anterior and posterior teeth has increased tremendously as they have high aesthetic potential with satisfactory durability and are less cost-effective.[16] Although composites are favourable restorative materials their polymerization shrinkage still remains a challenge. Various modifications are done in composite materials such as matrix and filler ingredients, filler particles size, and adhesive systems to further enhance the physical and mechanical properties. Fillers are active constituents that are responsible for most of the mechanical properties of the resin restorations.[17] Nanohybrid is a hybrid resin composite with improved distribution of fillers in the matrix by combining, Nanofiller in a pre-polymerized filler form together with submicron particles to achieve better mechanical, chemical, and optical properties.[3], [18]
The present study evaluated the effect of the addition of Graphene Nanoparticles on the Surface roughness, Microhardness, and Flexural strength of Nanohybrid composite resin (Tetric N Ceram) after subjecting the specimens to a thermomechanical loading cycle. The present study was strongly supported by previous studies in which improvements in mechanical properties were reported on the addition of various Nanoparticles.[6], [7], [19], [20], [21], [22] The Control group C was the unmodified composite resin, the two interventional groups are CG1 and CG2 with 0.01% and 0.02% w/w ratio Graphene Nanoparticles added to composite resin.
Graphene was first demonstrated in 2004 by Andre Geim and Konstantin Novoselov, two physicists from the University of Manchester, for which they received a Noble prize in 2010. The name Graphene is derived from “graphite” and the suffixing implies that it is an allotrope of carbon arranged in a two-dimensional honeycomb lattice Nanostructure. Pure graphene is in the form of Few-layer graphene (FLG -1 to 6 layers) which are held together by van der Waals forces. The desirable property of Graphene is that it can be cross-linked to various chemicals to establish both interlayer load transfer and intralayer load transfer (graphene layers are bridged on the edges, in the same plane).[9] They have unique, mechanical, and stress dissipation properties and are biocompatible and non-cytotoxic. Graphene has high mechanical strength and hence enhances the mechanical, physical, and chemical properties of biomaterials which makes Graphene Nanoparticles, an ideal material to incorporate into composite resin to increase their physicomechanical properties and durability for better clinical performance.[10] Though studies have reported that modifications of the resin composite by incorporation of various Nanoparticles there was not much literature about modifications by composite resin with Graphene nanoparticles though it is biocompatible with good mechanical properties.
The Surface roughness of restorative materials is directly related to wear, and discoloration. Rough surface causes accumulation of biofilms, food residues, and stains which can cause gingival irritation, and risk of secondary caries, affecting the gloss of the restoration, resulting in discoloration and/or surface degradation. The present study proved that varying percentages of Graphene nanoparticles added had a significant effect on the surface roughness of the specimens. According to studies, the roughness of the restoration can be detected by the tongue when the roughness value is above 0.5µm. [23], [24] In the present study, the surface roughness values of both control and interventional groups are less than 0.2 µm which makes the intervention more acceptable. The Control group had the least surface roughness value (0.154 ± 0.001) when compared to the interventional groups. Among the interventional groups the highest surface roughness values were demonstrated in the CG2 group (0.185 ± 0.003) and the lowest values in the CG1 group (0.165 ± 0.003). The reason for this could be that when a lower percentage of Nanoparticle (0.01% GNP) was added to the composite it could have dissolved completely in the monomer and there could be less filler loading in the outer surface of the restoration following finishing and polishing. CG2 demonstrated more surface roughness may be because of the presence of undissolved and agglomerated Nanoparticles. [25]
Microhardness is a parameter used to evaluate the surface resistance of restorative material to plastic deformation. Wearing of the restoration cause faulty tooth relationship which would affect aesthetics and masticatory efficiency. In the current study, the Vickers hardness test was applied to measure the Microhardness values of the composite resin specimens. The Control group had the least Microhardness value (60.3 ± 1.0) when compared to the interventional groups. Among the interventional groups the highest Microhardness values were demonstrated in the CG1 group (94.0 ± 2.5) and the lowest values in the CG2 group (85.5 ± 1.3). This result can be explained by presuming that smaller size and lesser concentration of GNP promotes close cross-linking of the nanoparticle to the resin particles thereby preventing their degradation. It could also be due to the new homogeneous surface between composite resin and nanoparticles when the concentration of graphene nanoparticles is more, the nanoparticles could have agglomerated and have been left without crosslinking to the resin matrix and hence reducing the Microhardness value.[25] The present study was in agreement with the previous study in which Mohammed Al Jafary, Jirun Sun, L. Lotfi reported an increase in surface Microhardness on adding TiO2 nanoparticles, Zirconia particles, and Acrylic acid-modified TiO2 nanoparticles into the resins composites. [7], [20], [22] Andreotti reported that the microhardness values of acrylic resins following incorporation of TiO2 nanoparticles gave similar results. 26 However, the study was in disagreement with the outcome of a reported study by Garcia where the microhardness of GICs was increased with a higher concentration (3%–5%) of TiO2 Nanoparticles. [26]
The flexural strength of a material is its ability to bend before it breaks just before its proportional limit. Flexural forces are the result of forces generated in clinical situations on the restorative material that might cause its permanent deformation. Flexural strength is evaluated by the three-point bending (ISO 9917 – 212). In the current study, all the groups tested had a flexural strength higher than 80 MPa, according to literature the Flexural strength value above this value justifies the use of the interventional group as a restorative material for occlusal restorations. [27] Among the interventional groups, the highest flexural strength values were demonstrated in the CG1 group (117.57 ± 6.46) and the lowest values in the CG2 group (77.55± 4.85). The mean flexural strength value for the control group C was 96.01 ± 4.73. The reason for the increased flexural strength of (0.01%) CG1 groups could be due to increased cross-linking between the nanoparticles and the composite. [28] Graphene is also capable of transferring stress across the interface thereby increasing the flexural strength of composite resin.[9], [10] There was a decrease in Flexural Strength in the 0.02% concentrations CG2 group which may be because when the Nanoparticle content is increased there may be insufficient matrix to bond or presence of a specific amount of agglomeration of particles thus weaken the interfacial bond between the Nanoparticles and the resin matrix.[20], [27] The present study was in agreement with the previously reported by Ming Tian, Moszner N, Mohammad Atai, Gracia where incorporation of nanoparticles of TiO2, Zirconia, thermally sintered silica, Nano Fibrillar silicate to composite resin in lesser concentrations improved the FS of the resin and resins modified with higher concentrations of nanoparticles reduced the FS values.[6], [18], [21], [26] However, the study was in disagreement with a study where the authors found a decreased flexural strength of acrylic resin with TiO2 groups, regardless of the concentration. [29]
However, the study has a few limitations, this is an in-vitro study in which all the factors which contribute to the success or failure of restorations in a clinical situation cannot be evaluated. Anti-cariogenic property, flow property, durability, and stability of the graphene nanoparticles are other important factors to predict their success in clinical practice, these properties of graphene nanoparticles are yet to be evaluated. The Colour of Graphene is a major disadvantage that affects aesthetics, restricting its use as core material and for the restoration of posteriors.
Conclusion
Graphene nanoparticles were added to Tetric N Ceram Nano Hybrid Composite resin. The interventional group CG1 containing 0.01% Graphene nanoparticles demonstrated enhanced Microhardness value and flexural strength value. The study concludes that incorporation of the Graphene Nanoparticles at a lesser amount of 0.01% would positively improve the physical and mechanical properties of the composite so, that it could be used clinically as a novel biomaterial with excellent biocompatibility and good mechanical properties to be used as a core material and as a posterior restorative material
Conflict of Interest
The authors declare no relevant conflicts of interest.
Source of Funding
None.
References
- R Gupta, AK Tomer, A Kumari. Bulkfill flowable composite resins - A review. Int J Appl Dent Sci 2017. [Google Scholar]
- D C Sarrett. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater 2005. [Google Scholar]
- R George. Nano composites - a Review. J Deny Oral Biosc 2011. [Google Scholar] [Crossref]
- S Sachdeva, P Kapoor, A K Tamrakar, R Noor. Nano -Composite Dental Resin: An Overview. Ann Dent Spec 2015. [Google Scholar]
- MM Karabela, ID Sideridou. Synthesis and study of properties of dental resin composites with different nanosilica particles size. Dent Mater 2011. [Google Scholar] [Crossref]
- M Tian, Y Gao, Y Liu, Y Liao, NE Hedin. Fabrication and evaluation of Bis-GMA/TEGDMA dental resins/composites containing nano fibrillar silicate. Dent Mater 2008. [Google Scholar]
- Mohammed Al Jafary, Mohamed Ibrahim Hashem, A Majdah, Al, Khandhari. Effect of Nanoparticles on Physico-MechanicalProperties of Flowable Dental Composite Resins. Sci Adv Mater 2019. [Google Scholar] [Crossref]
- OK Hepdeniz, RB Ermis. Comparative Evaluation of Marginal Adaptation and Microleakage of Low shrinking Composites after Thermocycling and Mechanical Loading. Niger J Clin Pract 2019. [Google Scholar]
- Si Malik, FM Ruddock, AH Dowling, K Byrne, W Schmitt, I Khalakhan. Graphene composites with dental and biomedical applicability. Beilstein J Nanotechnol 2018. [Google Scholar] [Crossref]
- S Sava, M Moldovan, Codrutasarosi. Effects of graphene addition on the mechanical properties of Composites for dental restoration. Materiale Plastice 2015. [Google Scholar]
- D M Abdel-Hamid, AMK Esawi, I Sami, R Elsalawy. Characterization of Nano- and Micro-Filled Resin Composites Used as Dental Restorative Materials. 2008. [Google Scholar]
- KS Vandewalle, HW Roberts, FA Rueggeberg. Power distribution across the face of different light guides and its effect on composite surface Microhardness. J Esthet Restor Dent 2008. [Google Scholar] [Crossref]
- Y Li, H Lin, G Zheng, X Zhang, Y Xu. A comparison study on the flexural strength and compressive strength of four resin modified luting glass ionomer cements. Biomed Mater Eng 2015. [Google Scholar] [Crossref]
- RD Paravina, L Roeder, H Lu, K Vogel, JM Powers. Effect of finishing and polishing procedures on surface roughness, gloss and color of resin-based composites. Am J Dent 2004. [Google Scholar]
- U Yap, KW Lye, CW Sau. Surface characteristics of tooth-colored restoratives polished utilizing different polishing systems. Oper Dent 1997. [Google Scholar]
- N Cramer, J Stansbury, C Bowman. Recent advances and developments in composite dental restorative materials. J Dent Res 2010. [Google Scholar]
- R Wang, E Habib, XX Zhu. Evaluation of the filler packing structures in dental resin composites: from theory to practice. Dent Mater 2018. [Google Scholar]
- N Moszner, S Klapdohr. Nanotechnology for dental composites. IJNT 2004. [Google Scholar] [Crossref]
- Y Xia, F Zhang, H Xie, N Gu. Nanoparticle-reinforced resin-based dental composites. J Dent 2008. [Google Scholar]
- J Sun, MA Forster, PM Johnson, N Eidelman, G Quinn, G Schumacher. Improving performance of dental resins by adding titanium dioxide nanoparticles. Dent Mater 2011. [Google Scholar] [Crossref]
- M Atai, A Pahlavan, N Moin. Nano-porous thermally sintered nano silica as novel fillers for dental composites. Dent Mater 2012. [Google Scholar] [Crossref]
- L Lotfi, J Javadpour, MR Naimi-Jamal. Biological and nano-indentation properties of polybenzoxazine-based composites reinforced with zirconia particles as a novel biomaterial. Biomed Mater Eng 2018. [Google Scholar] [Crossref]
- CM Bollen, P Lambrechts, M Quirynen. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent Mater 1997. [Google Scholar]
- M Quirynen, C M Bollen. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J Clin Periodontol 1995. [Google Scholar]
- Y Han, Y Zhao, C Xie, JM Powers, S Kiat-Amnuay. Color stability of pigmented maxillofacial silicone elastomer: Effects of nano-oxides as opacifiers. J Dent 2010. [Google Scholar]
- R Garcia-Contreras, RJ Scougall-Vilchis, R Contreras-Bulnes, H Sakagami, RA Morales-Luckie, H Nakajima. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J Appl Oral Sci 2015. [Google Scholar]
- H Lu, YK Lee, M Oguri, JM Powers. Properties of a dental resin composite with a spherical inorganic filler. Oper Dent 2006. [Google Scholar]
- P Harini, K Mohamed, T Padmanabhan. Effect of titanium dioxide nanoparticles on the flexural strength of polymethylmethacrylate: An in vitro study. Indian J Dent Res 2014. [Google Scholar]
- AM Andreotti, MC Goiato, A Moreno, AS Nobrega, AA Pesqueira, DM Santos. Influence of nanoparticles on color stability, Microhardness, and flexural strength of acrylic resins specific for ocular prosthesis. Int J Nanomedicine 2014. [Google Scholar] [Crossref]
- Introduction
- Materials and Methods
- Preparation of experimental composite
- Specimen preparation
- Preparation of specimens for Surface Roughness (SR and Microhardness (MR
- Preparation of Specimen for Flexural Strength (FS)
- Thermal & mechanical loading cycling
- Specimen testing
- Results
- Discussion
- Conclusion
- Conflict of Interest
- Source of Funding
How to Cite This Article
Vancouver
S M, Chakravarthy D, S V. Evaluation of physico -mechanical properties of graphene nanoparticles modified nano hybrid composite resin after thermo mechanical loading cycle - An in vitro study [Internet]. IP Indian J Conserv Endod. 2022 [cited 2025 Sep 10];7(2):61-66. Available from: https://doi.org/10.18231/j.ijce.2022.013
APA
S, M., Chakravarthy, D., S, V. (2022). Evaluation of physico -mechanical properties of graphene nanoparticles modified nano hybrid composite resin after thermo mechanical loading cycle - An in vitro study. IP Indian J Conserv Endod, 7(2), 61-66. https://doi.org/10.18231/j.ijce.2022.013
MLA
S, Madhumita, Chakravarthy, Dhanavel, S, Viajayaraja. "Evaluation of physico -mechanical properties of graphene nanoparticles modified nano hybrid composite resin after thermo mechanical loading cycle - An in vitro study." IP Indian J Conserv Endod, vol. 7, no. 2, 2022, pp. 61-66. https://doi.org/10.18231/j.ijce.2022.013
Chicago
S, M., Chakravarthy, D., S, V.. "Evaluation of physico -mechanical properties of graphene nanoparticles modified nano hybrid composite resin after thermo mechanical loading cycle - An in vitro study." IP Indian J Conserv Endod 7, no. 2 (2022): 61-66. https://doi.org/10.18231/j.ijce.2022.013
